Metal-organic frameworks are a sub-class of coordination polymer and defined as repeating units of metal ions, or clusters, linked by organic molecules. These organic molecules are often multidentate, such as the hexadentate 5,5’,5’’-(1,3,5-triazine-2,4,6-triyl)tris(azanediyl)triisophthalate (TATAT) and 1,4-benzezedicarboxylate (BDC).5,6
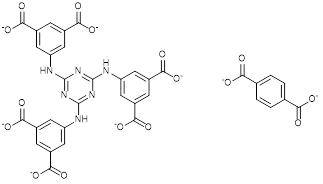
Fig. 1: TATAT (left) and BDC ligands
The vast choice of ligands available to groups undertaking research in this field allows for constant adaptation to each MOF with the simplest of changes to the procedure. These changes can have varying effects on the properties of the frameworks synthesised, from surface area to cavity volume.1
In addition to synthesising MOFs of differing sizes, there has been a recent surge in research towards the synthesis of amorphous MOFs. While the research into crystalline MOFs has been successful, Cheetham et al. and Fairen-Jimenez et al., amongst others, were keen to explore methods of improving the chemical properties of MOFs that were considered unsuccessful in the crystalline forms.6,7
These easily tuneable properties mean that MOFs can be used in drug delivery, catalysis, gas separation, and gas storage.8,9 To increase the affinity and selectivity of a MOF towards a certain gas or reactant, the linker molecules can be changed to tune the topology and cavity size of each framework.4,8,9
